Looking for a better understanding of electronic consent?
Managing consent for research projects is easier with electronic consent, which can boost participation and improve cost-efficiency.
It makes associated admin tasks easier for legal professionals too, and there are also benefits for research subjects.
Learn everything you need to know about it in this article.
Key takeaways
- Electronic consent can increase participation in research.
- eConsent is an effective tactic for managing the costs of administering research projects.
- That said, it presents several legal and ethical issues that you should consider.
- There are different kinds of eConsent, and some are better than others.
What is eConsent, and when is it used?
Electronic consent (eConsent) is a method of obtaining agreement from an individual. It’s also known as electronic informed consent (eIC).
For example, medical researchers must obtain consent for trials of new therapeutic drugs or devices.
Legal professionals will likely undertake this task, and they must inform medical research subjects of any risks involved in their participation.
The key differentiator with eConsent compared to standard consent is that it’s electronic.
Agreement is sought via digital means rather than in written form.
This means you must employ the use of electronic signature software, which allows you to securely gain verifiable consent from participants.
It’s important to remember that subjects must understand what they’re consenting to.
This information can be passed on through digital media, such as online videos, articles, audio, and interactive learning modules.
So, when is electronic consent used?
Well, it’s useful for subjects located in a variety of places, as it means participation needn’t be limited by how close people are to the center of research.
They can learn about and consent to their participation in it remotely.
What are the benefits of eConsent?
Research requires a lot of data to be valuable.
This can be particularly challenging in the field of medical research.
Drugs are often developed to treat a certain condition, so researchers need a pool of patients with said condition.
However, there may not be a lot of suitable candidates located close to the research site. In this case, researchers must look further afield.
They still need to get consent no matter how far away participants are.
One benefit of electronic consent is that they can reach anyone with internet access.
This is more convenient for the patient and researcher.
It also creates an opportunity to widen the subject pool as geography becomes less of a factor.
Of course, researchers must consider costs as well as the value of their scientific endeavors.
Subjects can usually expect to be reimbursed for their travel to the research center. With electronic consent, you can reduce expenses.
There are benefits for research subjects too.
You can give them more time to absorb the information — something that isn’t always possible if the subject is on-site with a paper form in front of them.
This can sometimes mean they feel pressured to sign without fully understanding the relevant information.
Specifically, you can bring eConsent forms to life with video content and interactive elements, which help potential subjects better absorb important information.
There are benefits for legal professionals and researchers — for example, you can manage consent documents online and in a secure environment.
You can also store them in a searchable database that’s accessible from anywhere in the world.
What are the issues presented by electronic consent?
Despite the upsides, there are certain ethical and legal issues to consider when relying on electronic consent.
In this section, we’ll explore some of these challenges.
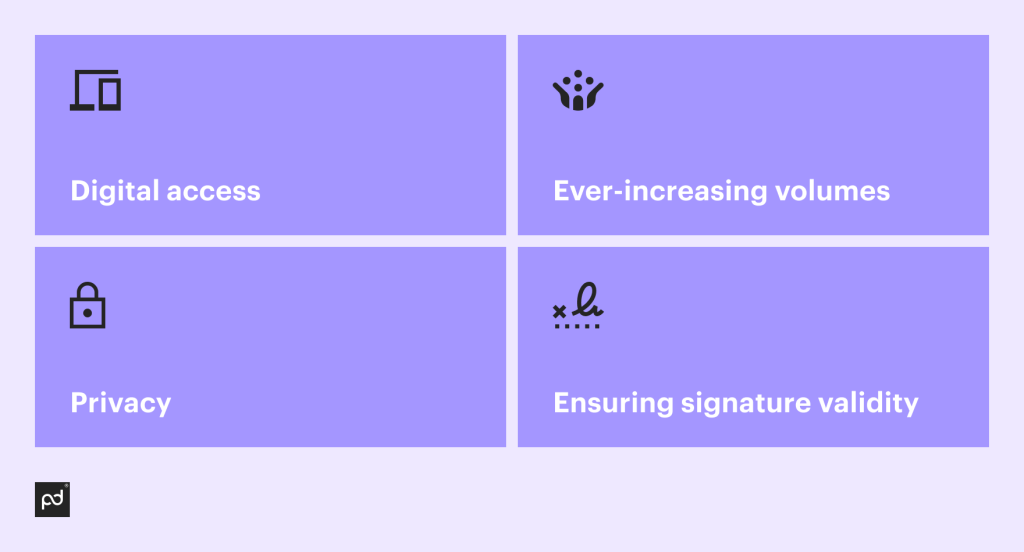
Digital access
Older generations tend to be wary of digitalization.
The same can be said of other groups with less computer literacy.
This includes subjects from rural backgrounds with less access to digital technologies, and such groups will need more guidance if you wish to use electronic consent.
Ever-increasing volumes
Electronic consent allows for increased participation in research, and this is good for science and medicine, especially genetics.
Genetic researchers can study samples from thousands of individuals, broadening their knowledge of a plethora of diseases and conditions.
Using electronic consent in this kind of research thus seems logical. With such large sample sizes, it makes sense to manage consent via digital means.
However, because of the issues around digital access, this may not be a good idea.
Ironically, it can lead to less diverse samples — for example, some minority groups have less digital access than the general population.
Privacy
Participants may also be concerned about privacy.
This is understandable when you consider how large data breaches often make the headlines.
That said, this challenge can be overcome by taking cybersecurity seriously.
Use encryption and password protection and let participants know about your various safety protocols.
Tell them who’ll have access to their data, too, and how they’ll use it.
Ensuring signature validity
Signatures must be verified in order to be legally compliant.
This is easy with paper forms and witnesses present. With electronic consent, it’s slightly more challenging.
However, signature verification is made possible with the use of certain software.
If you go down this route, you must ensure you choose an option that’s compliant with any relevant legislation.
What are the different types of electronic consent?
When providing electronic consent online, there are a variety of ways to do it. Let’s examine them now.
Scanned
This is where subjects write their signature by hand and scan it into a computer.
The signature then becomes a common image file format, such as a jpeg.
The subject can insert this into the appropriate section of the document.
Typed
The individual types their name into the form, and is then required to tick a box confirming consent.
eSignature
Put simply, it’s a secure form of electronic consent. It uses encryption to create a unique digital signature when a user signs a document.
Stylus
A stylus is a pen-like device you can use to handwrite or hand draw on a computer.
You can use it on a touch screen or via a specialized pad. A subject will need one of these devices on hand to give consent in this way.
What to include in an electronic consent form
The project you’re seeking consent for may be covered by various legislation, so make sure to do your homework before you draft your consent form.
In general terms, consent forms should avoid the use of overly technical language.
If you look at an example, you’ll see they’re generally written using simple wording.
This should be as comprehensible as possible for people who aren’t experts.
General advice is to avoid any wording that could be seen as coercive or confusing, and, most importantly, you must not make false or misleading statements.
You might want to use an electronic consent form template to make life easier.
This will generally include some standard content, such as:
- Stating that the project involves research. Make clear that the relationship is between a subject and researcher versus a doctor and patient.
- The purpose of the research. You should state the reasons for conducting this research and what the researchers are hoping to achieve.
- What’s expected of the participant. Use this section to explain what the subject will need to do to take part. Include how long you expect the participant to be involved.
- Risks. List all known possible risks associated with participation.
- Benefits to the subject. Explain how the participant will benefit from taking part.
- Costs. Explain what will and will not be covered by insurance.
- Alternative therapies. Where other treatments exist, you should explain them here.
- A statement explaining that participation is voluntary. You must make clear that the subject is under no obligation to participate.
- Subjects can withdraw consent at any time. You must also state that participation can end at the subject’s discretion. There can be no penalty for doing so.
- Confidential. Explain what the researchers will do to ensure confidentiality. Show how the research is covered by HIPAA rules.
- Why researchers may terminate the subject’s participation. Explain the circumstances under which the subject may be asked to leave the project.
- Contact information. A section of the consent form should be dedicated to listing contact information, so participants know where to direct questions.
- Signatures. There should also be somewhere for the participant or their legal representative to sign e.g. to add a qualified electronic signature.
Meet all your eConsent requirements with PandaDoc’s eSignature software
You can use electronic consent to increase participation, cut costs, and improve administrative efficiency, but it’s vital you do this in a secure way.
That’s where PandaDoc comes in.
PandaDoc’s eSignature software protects your subjects’ privacy.
It’s not only HIPAA-compliant but legally binding, with each and every eSignature coming with a unique digital certificate. This means you and your subjects can be confident in the consent process.
What’s more, it has an extensive library of document templates for you to choose from.
Consent forms, contracts, and research proposals can all be created online with PandaDoc.
Managing these documents is easy too, and you can access them from anywhere in the world.
Why not start a 14-day free trial today?
Disclaimer
PandDoc is not a law firm, or a substitute for an attorney or law firm. This page is not intended to and does not provide legal advice. Should you have legal questions on the validity of e-signatures or digital signatures and the enforceability thereof, please consult with an attorney or law firm. Use of PandaDocs services are governed by our Terms of Use and Privacy Policy.