Good documentation practices (GDocP) consists of a set of guidelines for creating, maintaining, and managing documents.
While generally associated with the research and development of pharmaceuticals and medical devices, this set of standards isn’t confined to a specific industry.
Organizations in any business or government sector — IT, legal services, software development, etc. — benefit from adhering to the principles and structure that drives GDocP.
At its core, GDocP aims to ensure the integrity and accuracy of information.
It guides how data should be recorded, stored, and retrieved, ensuring that every piece of information is traceable, reliable, and easily accessible.
This includes everything from the creation of original records to their modification, distribution, and eventual archiving or disposal.
In this article, we’ll detail how the FDA’s ALCOA+ framework is interlinked with having strong GDocP standards in place, and share a journey of establishing and maintaining good documentation practices.
Finally, we’ll widen out, addressing principles of good documentation, as well as find out how it can revolutionize your operations.
Key takeaways
- Data integrity plays a pivotal role in effective documentation practice. The ALCOA+ framework is essential for data integrity in life sciences, ensuring that data abides by the following criteria: Attributable, Legible, Contemporaneous, Original, Accurate, Complete, Consistent, Enduring, and Available.
- Principles of good documentation refer to accuracy, clarity, timeliness, traceability, consistency, legibility, authorization, version control, protection, retention, training, validation, review cycles, risk-based approach, root cause analysis, and continuous improvement.
- Electronic records can revolutionize information management with increased speed, vast storage, and efficient solutions, but also pose challenges to data security and integrity.
- GDocP includes the use of a document management system (DMS), multilevel auditing, random document sampling, correlation of product non-conformances, training of auditors, and establishing self-sustaining documentation systems.
- Strategic steps align documentation with regulatory requirements and business goals. These include setting SMART objectives, KPI tracking, risk-based gap assessments, rotating document review responsibilities, closed-loop CAPA system, effective training, and regular review and updates.
FDA definition of good documentation practices
The Food and Drug Administration (FDA) provides a clear definition of good documentation practices in the context of the pharmaceutical and medical device industries.
According to the FDA, GDocP is the set of standards by which documents are created, modified, maintained, and archived.
(To avoid confusion, it’s worth saying that the acronym GDP means good distribution practice, a term from the European Medicines Agency, which covers the aspects of human medicine distribution, from purchasing active pharmaceutical ingredients to selling medicinal products to consumers.)
The definition of documents here is somewhat broader. They include:
- written procedures,
- work instructions,
- forms,
- records,
- logs,
- labels,
- electronic documents,
- laboratory notebooks,
- emails, and even more.
Essentially, any piece of information that records or conveys an action, decision, or event within the organization can be considered a document.
Difference between the principles of good documentation, good documentation practices, and a good document
Understanding the difference between the principles of good documentation, good documentation practices, and a good document is crucial for effective information management.
Here’s an illustrative breakdown:
- Principles of good documentation is the widest and most theoretical term, meaning the fundamental guidelines that underpin all good documentation efforts. They are the “why” behind the practice and the qualities of a good document. For example, the principle of accuracy underscores the importance of correct information, which is crucial for making informed decisions.
- Good documentation practices (GDocP) are the above-defined methods or procedures that adhere to the principles of good documentation, making them a nested component of these principles. GDocP is the “how” of the process. For instance, a good practice could be using a standardized template for all meeting minutes to ensure consistency.
- A good document is the end product of applying the principles through good documentation practices. It’s the “what” that results from the process. A good document is accurate, consistent, accessible, secure, and compliant. For example, a well-drafted contract that accurately reflects the agreement follows an established format, is easily retrievable, securely stored, and complies with relevant standards would be considered a good document.
16 principles of good documentation
The list below represents the principles of good documentation, fundamental guidelines expanding beyond what can be found in good documentation practices.
These principles are universal; as such, each one is illustrated by a relevant example for clear and unambiguous understanding so you can visualize implementing them in your company or workplace environment.
1. Accuracy
Every piece of information recorded should be correct and precise.
This ensures that the document serves as a reliable source of information for decision-making, process improvement, and regulatory compliance.
Going practical:
- Implement a data validation checklist covering key details like timestamps, author initials, units of measure, references, calculations, etc.
- Require sign-off from a second reviewer.
Example:
A biotechnology firm faced regulatory attention due to inconsistencies in its research data. To rectify this, they initiated a comprehensive training program, reaching out to 12,000 employees across 18 global locations. The training, conducted in eight different languages, emphasized the importance of accurate data recording, enhancing the firm’s credibility, and ensuring regulatory compliance.
2. Clarity and completeness
Documentation should be clear (unambiguous and not confusing), understandable, and complete. Use plain language, provide context, and include all relevant details.
Going practical:
Develop document templates that prompt authors to include all necessary details. Include fields for title, purpose, scope, definitions, people involved, processes covered, etc.
Example:
A company created clear, comprehensive, and contextually relevant educational materials. Company reps collaborated with local experts to customize the content, making it culturally and linguistically appropriate for each location. This approach ensured that every employee, regardless of their role or location, had a clear understanding of their responsibilities.
3. Timeliness
Documentation should be recorded without delays and kept up-to-date.
Untimely records can block other tasks and feed decision-makers with outdated information, decreasing the performance of specific employees and the whole company.
They also raise the chance regulatory agencies will catch you in non-compliance, and you’ll be unable to prove the opposite.
Finally, this can end up in missed opportunities, damaged reputation, and even legal jeopardy.
Going practical:
Implement a version control system that automatically timestamps all document edits. Require reviewers to verify timestamps before approving changes.
Example:
Answering the regulatory request, a company rolled out its training program within six months, as it was required. Such a punctual action demonstrated their commitment to regulatory compliance and their proactive approach to addressing issues.
4. Traceability and audit trails
Maintain document traceability by recording all the changes to your documents within a full lifecycle. To ensure this is possible, your technical writers should structure content to make information traceable and modifications easily tracked. This allows quality managers, auditors, and inspectors to conduct audit trails and make assessments of documentation integrity.
Going practical:
Tag all documents with metadata for easy tracking, including author, approver, date created, and status. Require this metadata to be filled in before any approval is issued.
Example:
A biomedical engineering firm implemented a robust version control system that allowed them to trace all changes made to their project plans. This practice was critical in maintaining the integrity of their documentation and facilitating root cause analysis in case of any discrepancies.
5. Consistency and standardization
Establish and use consistent formats, templates, and terminology across documentation whenever possible.
To make it efficient, your document controllers should ensure other employees follow the standards you’ve enacted.
You should also add learning of relevant documentation standards to educational programs for personnel.
Going practical:
Customize document templates for each type of record with standardized sections, headings, fonts, and terminology. Make the templates mandatory for all authors.
Example:
A multinational corporation standardized its internal communication templates. As a result, they reduced human errors, improved performance, and ensured that all employees, regardless of their location, received consistent information.
6. Legibility
Carefully choose fonts, their sizes, leadings, the contrast ratio between the background and text, layouts, and other design parameters which can enhance the physical readability of your documents. Assign technical writers and document controllers responsible for ensuring that content is written for maximum legibility.
Going practical:
- Select easily readable font styles and minimum font sizes based on legibility research.
- Conduct user tests to optimize font sizes for various readers and mandate their use.
Example:
An eye clinic’s network developed patient information leaflets for their services. The leaflets were designed with enlarged fonts and high contrast ratio, ensuring that patients with visual impairments could quickly obtain all the relevant information about their care.
7. Authorization, approval, and accountability
Only authorized individuals should approve documents and be accountable for the accuracy of generating and storing documentation.
Regulatory compliance professionals must rely on document approvals and accountable persons to be able to prove the company’s commitment to quality and compliance in case of regulatory agency assessment.
Going practical:
- Implement a system of document sign-offs at multiple stages of development.
- Track all sign-offs and assign accountability to specific roles within the documents.
Example:
A financial institution implemented into their computer system a mandatory authorized-only access to sensitive documents. Shortly thereafter, new cases of unauthorized access or alterations dropped to zero.
8. Version control and change management
By keeping your documents up-to-date and maintaining a history of all their changes, you can implement efficient version control and change management processes.
This will help quality assurance professionals ensure that the documents in the company meet regulatory requirements.
Going practical:
Adopt version control software that manages document changes, archives all versions, and rolls back if needed. Enforce change control procedures that define who can approve changes.
Example:
A medical software development company used a version control system to maintain up-to-date versions of their code. Since the system was implemented, there were no new issues related to undocumented code changes, and mean time to repair (MTTR) has dropped threefold.
9. Protection, security, and restricted access
Documents containing sensitive information must be reliably protected from unauthorized access, alteration, and destruction.
Assign information security personnel and document controllers responsible for implementing security measures and access controls to safeguard documentation.
Going practical:
Classify documents based on sensitivity. Enforce varying levels of access controls and data encryption for different document classes. Restrict access to authorized personnel only.
Example:
A pharmaceutical research company implemented stringent security measures to protect its internal knowledge base. They used encryption and access controls as preventive actions against unauthorized access or alteration of their documentation.
10. Retention and destruction
Hold your documents only for the required period and destroy them securely when no longer needed.
Regulatory compliance professionals must develop schedules for documentation retention and destruction, and enforce them in order to mitigate the high risk of noncompliance.
Going practical:
Create a document retention schedule that defines retention periods for each document type based on legal and compliance needs. Implement secure deletion practices aligned with regulatory requirements.
Example:
A law firm adhered to strict policies for the retention and destruction of client files. Setting the required period of retention and arranging the secure destruction of documents when no longer needed, they secured both client confidentiality and regulatory compliance.
11. Training and awareness
Train your relevant personnel on practices of good documentation and ensure they’re aware of why compliance is important.
Assign responsible staff members as training professionals to control the coverage of good documentation practices and data integrity requirements in training programs.
Going practical:
Develop role-specific checklists and e-learning modules covering responsibilities, required practices, and sample scenarios. Test knowledge with assessments and refresher training.
Example:
A drug manufacturer* conducted regular training sessions on practices of good documentation for its back office. Their reps emphasized the importance of compliance and the consequences of noncompliance and set everything up and running to educate employees on their additional responsibilities.
*In terms of manufacturing, there is the term GMP, which means good manufacturing practice — a set of rules which manufacturers in regulated industries must follow in order to make their medicinal products safe, pure, and effective.
Over time, GMP emerged as cGMP (current good manufacturing practice), referring to the fact that drug manufacturers must use only up-to-date technologies to comply with GMP regulations.
As a result, GMP became a much broader term in the US, referring to the rules of consistent production and quality control in accordance with standards for manufacturers across various industries.
Outside the US, these two terms mostly remain interchangeable.
12. Validation
Validate any systems your company uses for generating or storing documentation for data integrity. Instead of relying on a vendor’s promises, entrust your information technology or information security personnel to conduct compliance validation at least once before starting to work with a new system.
Going practical:
- Validate your document management system for FDA 21 CFR Part 11 compliance before implementation.
- Conduct periodic risk-based assessments and system revalidations.
Example:
A pharmaceutical company decided to validate an electronic documentation system before usage. This resulted in minor data corruption, of which a vendor wasn’t even aware. After receiving a report, the vendor fixed corruption quickly and granted the company a substantial discount.
13. Review сycles
Regularly review your documents to spot and correct errors, verify their relevance, and ensure compliance.
Assign quality assurance and regulatory compliance professionals to conduct these reviews to facilitate this process. Regular reviews help to maintain the accuracy and reliability of your documents.
Going practical:
Establish standard review cycles for different document types. Rotate review responsibilities across different teams. Track review results to identify areas for improvement.
An insurance company conducted regular reviews of their policy documents. They identified and corrected errors, ensured relevance, and verified compliance, maintaining the accuracy and reliability of their offerings.
14. Risk-based approach
Prioritize your good documentation practices based on risk to focus your resources where they can make the most impact.
You first should identify potential risks, then assess the probability and potential impact of each one.
Risks with the highest probability of occurrence and with the largest potential impact get the highest priority.
The last step is allocating resources in order to focus your time, money, and effort on mitigating the highest-priority risks first.
Going practical:
- Define a formal risk assessment process that includes identifying, assessing, and prioritizing risks based on likelihood and impact.
- For high-priority risks, implement phased risk control procedures, starting with the easiest, fastest solutions.
Example:
A leading cybersecurity firm adopted a risk-based approach to prioritize risks. Within risk assessment, they evaluated priorities for unauthorized access to sensitive documents, loss of critical data due to system failures, and non-compliance with data protection regulations. Sensitive document access got the highest priority, so they allocated resources to implement stringent access controls and robust encryption methods.
15. Root cause analysis
By conducting a root cause analysis, you can identify and address the underlying causes of documentation problems rather than just treating the symptoms.
The analysis starts with a clear identification of the problem, followed by collecting data related to the problem, continues with the identification of patterns or common factors, and finishes with the detection of underlying causes of the problem and then develops a suitable solution based on the findings.
Going practical:
- Recommend a structured root cause analysis methodology with sequentially implemented steps.
- Focus on defining concrete responsibilities, timelines, and controls around risk mitigation and issue resolution.
Example:
A medical device manufacturing company faced recurring issues with their assembly instructions documentation. The company initiated a root cause analysis and reviewed the problematic assembly instructions, and interviewed the creators and production floor staff who used them. They discovered that the technical writers were not adequately familiar with the assembly process and that the production staff lacked sufficient training on how to interpret and apply the instructions. The issue was solved by providing pieces of training for both writers and floor staff.
16. Continuous improvement
Identify and implement improvements to your documentation processes over time to maintain ongoing compliance.
Quality managers should establish systems to identify opportunities for improvement and track the progress of these improvements.
Going practical:
Conduct periodic surveys to solicit employee feedback. Implement iterative, experimental improvements and measure their impact.
Example:
A logistics company identified and implemented improvements to its documentation processes over time. They established a system to track the progress of improvements, ensuring ongoing compliance and efficiency.
The role of ALCOA+ in good documentation practices
This exploration is a practical guide designed to empower professionals to harness the power of effective documentation, propelling their businesses toward greater success.
The ALCOA+ framework is a set of principles focused on data integrity in the life sciences sector, introduced by the FDA.
This is a fundamental part of data integrity in various good practice (GxP) guidelines.
Whereas GxP is a general term for good practice quality guidelines and regulations in various fields, ALCOA+ is explicitly associated with various industry-wide guidelines for:
- Pharmaceuticals and other manufacturing niches within good manufacturing practice (GMP);
- The range of industries where good documentation practices (GDocP) applies, including audit trails and data entry systems as its integral parts;
- Laboratories within good laboratory practice (GLP), particularly covering laboratory notebooks as the core component of GLP**;
- Clinical trials within good clinical practice (GCP***);
- Other existing GxPs****;
- Raw data management, and more.
**GLP, an acronym for good laboratory practice, is a system of quality assurance covering research (non-clinical) laboratories.
***For regulating clinical trials (those involving human subjects), a similar set of guidelines and standards is called GCP (good clinical practice).
****GxP is an overarching term for different kinds of good practice.
The ALCOA acronym without a plus sign means five principles — Attributable, Legible, Contemporaneous, Original, and Accurate. Next came the remaining four principles — Complete, Consistent, Enduring, and Available. Let’s look at each of the principles in more detail:
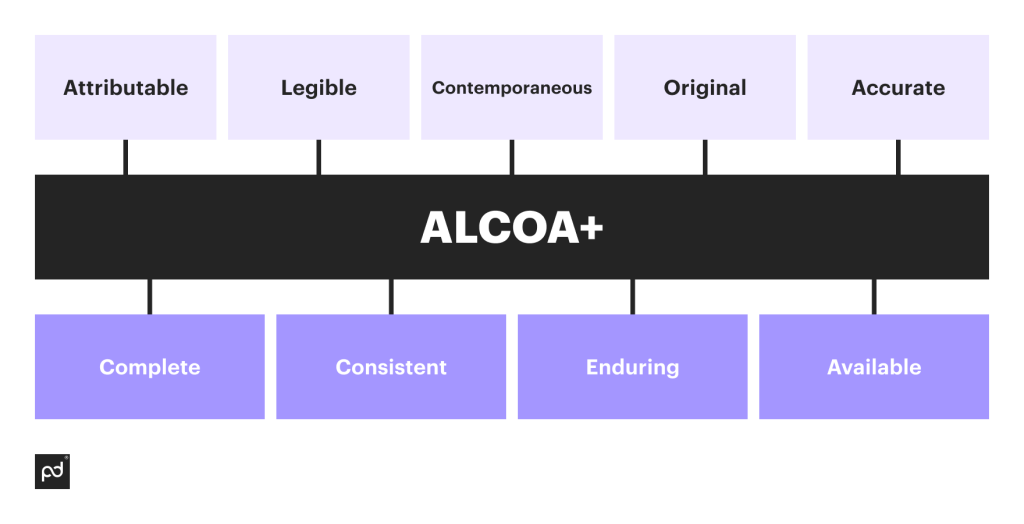
Attributable
The principle of attribution requires that every piece of data be traceable back to its originator.
Whether it’s a person, a system, a sensor, or a device, the identity of the data source must be recorded, along with the date and time of data collection, generation, or update.
This applies to both manually entered and automatically generated data.
Legible
Legibility is about ensuring that data is clear, readable, and comprehensible, regardless of whether it’s handwritten or digitally generated.
The goal is to ensure that the data remains understandable for years or even decades after its creation, often meaning the usage of consistent, simple language across the organization.
Contemporaneous
This principle emphasizes the importance of recording data at the exact moment an activity or action occurs.
In the case of electronic data, this often involves automatic timestamping but requires taking care to avoid any queuing delays that could affect the accuracy of the timestamp.
Original
The originality principle stipulates that the primary record should be the original data recording, whether it’s on paper or within a digital system.
Copies or transcriptions should not replace the original record.
Accurate
Accuracy in data is non-negotiable.
Data must be correct, precise, and free from errors to support informed decision-making and ensure regulatory compliance.
Complete
Completeness requires that all data, including any associated metadata, be included in the record.
This ensures a comprehensive and reliable source of information.
Consistent
Consistency involves using uniform formats, templates, and terminology across all documentation.
This reduces the potential for errors and enhances efficiency.
Enduring
Endurance refers to the longevity of data.
Data should be stored in a format that remains unchanged and accessible for the duration of its required retention period.
Available
Availability means that data should be easily accessible for review or inspection when needed.
This is particularly important for audits and inspections.
We’ve now succinctly elucidated the essence of ALCOA+.
By adhering to these nine principles, it’s possible to enhance record-keeping practices significantly in a remarkably short period of time.
This, in turn, can transform your documentation process into an efficient, pivotal component of leveling-up your business operations.
The imperative of legibility and validation
In the realm of documentation, legibility, and validation are akin to the twin pillars holding up a structure.
They are fundamental to the creation of a document that not only serves its purpose but also enhances operational efficiency.
Legibility is more than just a measure of how easily text can be read. It’s the thread that binds the structure and content of the document together.
For instance, an IT user manual needs to be legible not just to the tech-savvy, but also to those who might struggle with technical jargon.
To check a document for legibility, you need to assess its structure, language, and use of visual aids.
Then you have to ensure that the document’s language is simple and that technical terms have proper definitions.
Validation, meanwhile, is the process of confirming the accuracy and reliability of the document’s content.
It’s the seal of approval that the information is not just correct, but also relevant and useful.
In the context of a contract, validation occurs when all parties have reviewed the terms and given their consent.
For validation, cross-check facts, verify data with reliable sources, and ensure compliance with relevant standards.
In the legal context, validation may require consent from all parties.
Harnessing the power of standard operating procedures (SOPs) and data integrity
Standard Operating Procedures, or SOPs, mean standardized processes designed to facilitate and speed up workflows.
They increase the efficiency of operations and lead all team members to follow the same consistencies in completing similar tasks.
Standard operating procedures help to make sure work is done correctly, consistently, and efficiently.
At the same time, data integrity plays a pivotal role at each step of the documentation journey, becoming the solid backbone of effective documentation practice.
Maintaining data integrity means making sure our information is correct and doesn’t change when it’s not supposed to.
Think of it as the “truthfulness” of our information.
Whether it’s customer details, sales records, or emails between staff, it’s important that this data is accurate and reliable for all.
To implement SOPs and achieve data integrity, follow these steps.
First, create clear, easy-to-follow instructions for your tasks — your SOPs.
Next, make sure you’re always checking the accuracy, non-alteration, and safety of your information.
Navigating electronic records, batch records, and regulatory requirements
Now that we’ve established a solid understanding of the FDA’s ALCOA+, it’s time to delve deeper into the intricacies of electronic records, batch records, and regulatory requirements, with practical insights and valuable pieces of advice.
Electronic records
These paperless records have revolutionized information management in several ways.
- They have significantly increased the speed of data processing and retrieval. Instant processing, in turn, has enabled real-time data analysis and decision-making.
- They’ve made it possible to store and manage vast amounts of data, which skyrocketed data analysis and business intelligence capabilities.
- Electronic records have become an efficient solution for managing large volumes of information with improved accessibility, factors that kickstarted the popularity of remote work and collaboration.
- However, electronic records posed massive challenges in terms of data security and integrity. These challenges can be addressed with advanced encryption, access controls, regular audits, and regular staff training on data security.
- Insight: Some data is frequently used while other data is rarely accessed but still needs to be retained for regulatory or business reasons. Implementing tiered storage can help optimize storage costs by storing frequently accessed data on high-performance storage and moving less frequently accessed data to lower-cost storage options.
Batch records
A batch record is a kind of specification detailing the process of manufacturing and distribution of a certain batch of specific products.
Such a strict framing allows companies to deliver consistent and stable products, which is crucial in the pharmaceutical sector and other industries.
- The process in batch records is described as detailed as possible and includes every aspect of manufacturing, starting from the identification and verification of raw materials used in the batch.
- Records also include specific actions taken, equipment used, its settings and specs, personnel involved in each step, deviations or issues with corrective actions taken, quality control results, and information about the release. Each page of the document must be signed by the person executing decisions and supervisor.
- Adding various in-process controls and testing makes batch records champions in terms of maintaining consistency and stability, as well as highly in-demand with respect to product fabrications sensitive to contingencies, like cosmetics, electronics, and chemical manufacturing.
- But the reverse side of the coin is that batch records are totally rigid — even minuscule change is always a big challenge.
Insight: An over-reliance on manual processes often leads to errors and inefficiencies. But implementing automation in such a predictable environment is logical and proven by the results and dividends that follow.
Regulatory requirements
These requirements are a complex set of rules and standards that companies must comply with.
These requirements can cover a wide range of industry-specific areas.
- Maintaining compliance with such complex regulations affects businesses mostly in a negative way, including high maintenance and monitoring costs, and additional spending on legal services.
- Avoidance of maintaining compliance with regulatory requirements, in turn, might be even more costly by non-compliance penalties, missed access to regulated markets, and reputational loss.
- When in compliance, regulatory requirements might give strong market players a competitive advantage, protecting their market share from weaker competitors.
- Insight: Regulatory requirements are often full of legal jargon that can be difficult to interpret. Hiring or consulting with a regulatory affairs expert is a valuable investment in this case. An expert can help interpret regulations, guide you on compliance implementation in a specific industry context, and help you stay one step ahead of regulatory changes. And the earlier you decide to accept professional help, the less likely you’ll be to waste money.
Advanced strategies in documentation management
Ensuring accurate and consistent records
This is a cornerstone of quality management and control within an organization.
Implementing the next strategic steps can lead to a robust documentation system that not only meets compliance requirements but also enhances efficiency and reliability.
So, at the start, we have a need for accurate and consistent record-keeping within an organization.
The integration of the steps detailed below creates a cohesive quality control strategy for identifying and fixing human errors, inconsistencies in records, and gaps in quality assurance.
Here’s a detailed exploration of the strategy we offer:
Step 1. Use a document management system (DMS) for automated syntax checks and spell checking
Leveraging a document management system can automate syntax and spell checking, ensuring that documents are free from common errors.
For example, a company can set up DMS to automatically flag and correct grammatical mistakes in standard operating procedures (SOPs).
This step is a basis for quality documentation practices, reducing manual effort and minimizing human error.
Step 2. Pair multi-level auditing with specialized role-based checklists
Automated checks catch only a subset of potential issues.
Pairing them with role-based checklists can help quality assurance personnel conduct multilevel audits that examine every aspect of the documentation process.
For instance, a quality manager might have a checklist focusing on compliance aspects, while a technical writer might focus on content accuracy.
This step adds an additional layer of scrutiny, enhancing the overall quality of the document control process.
Step 3. Integrate random document sampling into quality audits to maintain vigilance
Random document sampling in audits adds an element of unpredictability, ensuring that all documents, not just those flagged by automated checks, are subject to review.
For example, a monthly audit might randomly select 5% of all documents for a detailed review using role-based checklists.
This step perfectly ties in with the previous ones by maintaining consistency in the whole strategy and eliminating the potential weakness of automation.
Step 4. Correlate product non-conformances with documentation gaps to identify root causes
Linking the product issues you found with corresponding documentation allows you to identify the root causes of non-conformances.
For instance, if a product defect is traced back to a miscommunication in a manufacturing guide, the specific gap in documentation can be identified and corrected.
This step uses the information gathered through audits and checks from previous steps to drive continuous improvement.
Step 5. Train auditors to independently verify critical documents
Finally, none of these strategies would be effective without properly trained auditors.
Specialized training programs can equip auditors with the skills to assess complex documents, such as regulatory filings or technical manuals.
This final step ensures that the organization has the in-house expertise to maintain quality assurance and document control, creating a self-sustaining system.
Establishing self-sustaining documentation systems
An organization faces the challenge of maintaining documentation that is both compliant with regulatory requirements and aligned with business goals.
The ultimate aim is to establish a self-sustaining documentation system is a strategic endeavor that requires a comprehensive and interconnected approach.
Let’s review the steps of this holistic strategy:
Step 1. Define SMART objectives
Begin by setting Specific, Measurable, Achievable, Relevant, and Time-bound (SMART) objectives that align with both compliance requirements and business goals.
For example, reducing error rates in documentation by 15% within six months.
The documentation process must always have clear and attainable targets.
Step 2. Track key performance indicators
Once you’ve defined clear objectives, you need to set the tracking of KPIs such as error rate, revision frequency, and approval time, which correspond with these objectives.
Monitoring KPIs on a regular basis helps identify areas of improvement and ensures you’re aligned with the set objectives.
Step 3. Conduct risk-based gap assessments
Perform quarterly risk-based gap assessments to identify discrepancies between current and desired documentation practices.
A pharmaceutical company, for instance, might conduct a gap assessment to identify discrepancies between its current documentation practices and GMP standards.
Addressing high-risk gaps often yields the greatest benefits with respect to compliance and quality assurance
Step 4. Rotate document review responsibilities
Ensure an unbiased evaluation by rotating document review responsibilities inside the departments.
This practice creates a comprehensive understanding of the entire documentation system by different staff members.
Step 5. Implement a closed-loop CAPA system
A corrective and preventive action (CAPA) system helps in identifying, tracking, and resolving non-conformances, as well as in ensuring that these issues do not recur.
When one is identified, the CAPA typically requires immediate corrective action, as well as an analysis of the root cause of the problem to prevent it from happening again in the future.
Implementing a closed-loop approach means ongoing monitoring and verification to ensure the efficacy of actions taken in preventing the recurrence of the problem.
If the actions are found to be ineffective, the process begins again, creating a continuous cycle of improvement.
Step 6. Ensure effective training and awareness programs
Training programs focused on proper documentation procedures are vital.
Regular workshops and training sessions ensure that all employees are aware of the standards and procedures, enhancing the overall quality of documentation.
For example, a healthcare organization could conduct regular training sessions for its staff on the documentation requirements of HIPAA compliance.
Step 7. Regularly review and update quality objectives, metrics, and documentation systems
Continuous reviews and updates ensure that the documentation systems remain aligned with evolving compliance requirements and business goals.
Regular training and role-specific guidelines keep all personnel accountable for their part in maintaining the system.
Refresher training every 6—12 months is the best option to keep knowledge and skills up to date.
Wrapping up
The guide has elucidated the essential principles and methods for effective documentation.
From the foundational ALCOA+ framework to advanced documentation strategies in quality management, the guide offers a robust roadmap for professionals across various industries.
Emphasizing accuracy, consistency, legibility, and validation, our guide underscores the imperative of aligning documentation practices with regulatory requirements and business goals.
The integration of automated checks, multilevel auditing, role-based checklists, and continuous improvement initiatives create a self-sustaining system that not only ensures compliance but also propels businesses toward greater success.
The practical insights into electronic records, batch records, regulatory requirements, and the implementation of SOPs further enrich the understanding of the intricacies of documentation.
Use them to transform your documentation process into a pivotal component of your operations and achieve excellence in record-keeping practices.
By implementing our all-in-one document management software, you can create, manage, and store documents in a secure and accessible manner but also ensure that your documents adhere to the principles of good documentation.
With features like customizable templates, electronic signatures, and workflow automation, our tool makes it easier than ever to implement good documentation practices.
Ready to take your documentation practices to the next level?
Start your free 14-day trial today or book a demo to see how it can revolutionize your documentation processes.
Disclaimer
PandaDoc is not a law firm, or a substitute for an attorney or law firm. This page is not intended to and does not provide legal advice. Should you have legal questions on the validity of e-signatures or digital signatures and the enforceability thereof, please consult with an attorney or law firm. Use of PandaDocs services are governed by our Terms of Use and Privacy Policy.


